by Kai Friesecke, Erica Gralla, Nadia Lahrichi, Patti Gravitt
As presented at the 2019 Winter Simulation Conference
Abstract
Due to insufficient resources, arbitrary decision making, and other challenges implementing effective screening processes, the burden of global cervical cancer has fallen disproportionately on low and middleincome countries. Despite many countries adopting new modern diagnostic procedures, the implementation of these programs lags far behind the policy changes and risks failure during their early stages. To mitigate these risks in an ongoing implementation of new screening processes in the Iquitos region of Peru, we propose using discrete event simulation (DES) to model the initial roll-out of a proven screening process. The DES model will yield insights into potential resource utilization and appropriate staff hours for various performance (coverage) scenarios, and support stakeholders in making appropriate decisions as they resource the implementation of this new screening policy.
Introduction
According to statistics collected by the Pan American Health Organization, cervical cancer is the leading cause of cancer deaths among women in Peru. Until recently, Peru relied on cytology-based screening programs, which are challenging to implement effectively in resource-constrained settings, in part due to the complexity and high resource requirements of the steps in the screening process. An alternative approach involves screening for human papillomavirus (HPV) and treating on site. This methodology, adopted by the Cancer Control Program in Peru, can be implemented systematically during routine appointments, increasing the number of women screened. However, it is crucial to appropriately resource this new screening process. Low- to middle-income countries operate with a finite amount of resources, and should they be mismanaged, screening programs may perform poorly, leading to reduced enthusiasm for the new program and reversion to previously ineffective programs.
We are leveraging an ongoing implementation research study in Iquitos which engages screening stakeholders to understand implementation barriers and collaboratively develop a stakeholder-designed implementation plan (NIH R01-CA190366). The results from this study will be used to develop a process description and a corresponding simulation model that captures the stochastic nature of the process and looks at the impact on a variety of key performance indicators (eg. screening coverage, waiting time, loss to follow up). In future work, we will model alternative screening processes, such as the existing cytology-based process, to support decisions about policy along with implementation.
Approach
Figure 1 shows the process diagram that describes the HPV screen-and-treat process, which was developed from stakeholder meetings in Iquitos, Peru. Data collection for the Iquitos pilot project is already underway, and will be used to parameterize the model within the SIMIO modeling environment. Patients are tracked from the initial sampling, through the waiting period while their sample is batched and analyzed, and, if required due to positive test result, through treatment. Policy changes include, but are not limited to, changing staff hours at individual health centers or the central laboratory, changing the frequency that samples are batched and transported, and deciding the location and optimal number of treatment (TVT) centers. Model outputs enable calculation of several key performance indicators: (1) percent of the population screened; (2) percent of precancers treated; (3) losses to follow up; (4) patient waiting and total times; and (5) resource usage, measured in staff-hours, sample time in system, and testing materials used.
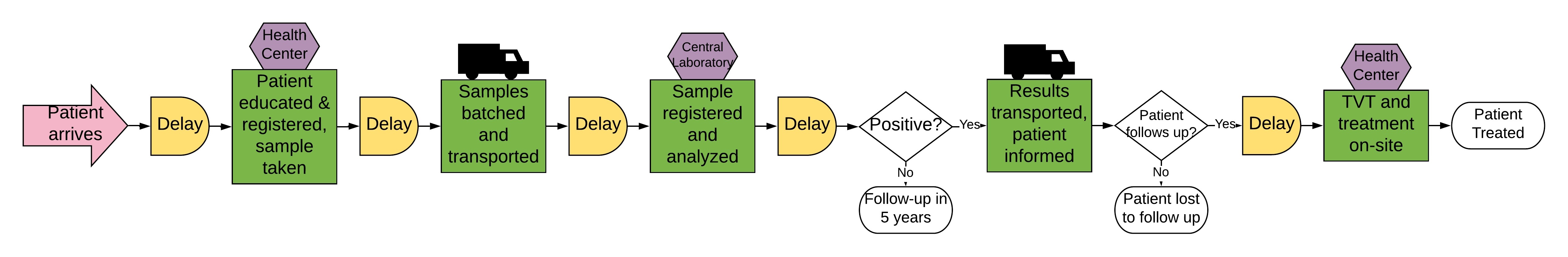
Figure 1: HPV screen and treat process diagram.
Results
Virtual experiments are conducted to determine the impact of different resourcing scenarios on the system’s performance. One example is observing the amount of time a sample will spend waiting prior to analysis at the central laboratory. Samples are not refrigerated, so all samples must be analyzed within 14 days. If the system targets sampling 33 samples a day across 17 health posts, batching weekly at noon on Wednesdays, the lab technician in the central laboratory must work at a minimum of 4 hours a day in order to achieve a maximum time in system of a sample of 13.29 days.
These results, and those of additional virtual experiments, are represented and interpreted to support stakeholder decisions on (1) the appropriate combination of resources to enable a given performance (e.g. number of women screened) and (2) the performance possible given a particular combination of resources. Stakeholders will thereby be able to understand the trade-offs between resource usage and performance and make better-informed decisions about the implementation of the new screening strategy.
Conclusion
The results support stakeholders in making data-informed decisions about resource requirements in resourceconstrained settings, and in particular in the Iquitos region of Peru. More generally, they underline the importance of appropriately resourcing the roll out of HPV-based screening programs in low- and middleincome countries. In future work, this framework can be expanded to the national scale and facilitate scaling-up of this pilot program throughout Peru and in similar middle-income countries.
